Curaleaf scrubs CBD health claims in response to FDA concerns
If Amazon doesn't have a Whole Foods grocery near you, there are non-perishable groceries ( food that doesn't spoil) that Amazon can ship to you
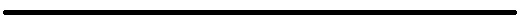
An employee speaks on the phone at a Curaleaf Inc. store in the Queens borough of New York, U.S., on Thursday, Oct. 18, 2018.
Jeenah Moon | Bloomberg | Getty Images
Curaleaf says it scrubbed its website and social media accounts of claims about its CBD products in response to a warning from the Food and Drug Administration saying it was making illegal health claims.
The FDA sent a warning letter to Curaleaf earlier this week, telling the cannabis company it was “illegally selling” CBD products with “unsubstantiated claims” that the products treat cancer, Alzheimer’s disease, opioid withdrawal, pain and pet anxiety.
Curaleaf in a press release Friday said upon receiving the letter, the company “immediately began an extensive review of its website and social media platforms to remove all statements that FDA identified as non-compliant.” The company said it removed its Curaleaf Hemp blog and third-party links in it, along with pulling statements and social media posts the FDA flagged.
“Curaleaf is committed to being an ethical and responsible company and working with the FDA to be a leader in our industry, setting the standards and guidelines to best service our customers and the communities we serve,” Curaleaf CEO Joseph Lusardi said in a statement.
Wakefield, Massachusetts-based Curaleaf sells CBD oils, lotions and pet products. CBD, short for cannabidiol, has been hailed as a treatment for a slew of ailments even though little data supports these claims. Consumer products containing CBD are not federally regulated, and the FDA has vowed to crack down on companies making “egregious” health claims.
The FDA is in the process of evaluating how it might regulate the booming CBD industry. The agency held a day-long meeting at its Silver Spring, Maryland, headquarters in May, where it grilled companies, advocates and scientists for any evidence that CBD actually does anything they claim it does.
A public comment hearing closed earlier this month. FDA Principal Deputy Commissioner Amy Abernethy in a Congressional hearing on Thursday said the agency received 4,492 comments. The plans to report on its progress early this fall.
 College Dorm and Apartment Cooking gadgets - if you change the sort settings on the Amazon page, it will show other items by price
College Dorm and Apartment Cooking gadgets - if you change the sort settings on the Amazon page, it will show other items by price
Source link

















